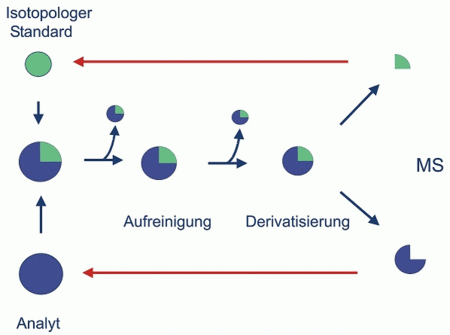
Principle
An isotopologic standard is added to the solution of the analyte and mixes with the latter during the equilibration stage. During clean-up and equilibration losses of analyte and standard occur, but without altering their molar ratio. Subsequently this ratio is analysed by mass spectrometry (MS). The amount of the analyte then can be calculated from the amount of added standard.