VITALAB – Exploring Fruit Byproducts and Whey for the Design of Innovative Foods with Increased Content of Vitamins Produced by Lactic Acid Bacteria
A sufficient supply of vitamin B9 (folate) is of great importance, especially for pregnant women, as a deficiency massively increases the risk of neural tube defects and other malformations of the fetus. In addition, an undersupply is potentially associated with the development of certain forms of cancer, Alzheimer's disease, and cardiovascular disease. Unfortunately, the folate intake worldwide is significantly lower than recommended. Different from Brazil and other non-European countries, a mandatory fortification is not implemented in Germany; as the supplementation with folic acid, relates to rising concerns about the unwanted side effects caused by the excessive intake of the synthetic form of the vitamin. Therefore, the purpose of the project VITALAB is to use selected folate-producing strains of lactic acid bacteria (LAB) to bio-fortify whey with folates by addition of fruit by-products. VITALAB started in spring 2020 as part of the initiative Bioeconomy International 2017 , which is jointly funded by the German Federal Ministry of Education and Research (BMBF) and the São Paulo Research Foundation (FAPESP). For a duration of 36 months this project will be conducted by the Department of Pharmaceutical and Biochemical Technology and the Department of Food and Experimental Nutrition at the School of Pharmaceutical Sciences, University of São Paulo (USP), São Paulo, Brazil and the Chair of Analytical Food Chemistry at the TUM School of Life Sciences, Technical University Munich (TUM)).
Fruit and milk processing is a huge industry in Brazil and significant amounts of side streams (e.g. fruit skin, yolk) are currently wasted. Therefore, these side streams will be used as substrates for LAB fermentation to optimize the folate production. Thus, this innovative procedure will be an interesting alternative to bio-fortify and increase the folate content in foods. By developing these products not only the dietary supply with this critical vitamin will be increased in Germany and Brazil, but also waste during food production and processing will be decreased. This supports the FAO “Global Initiative on Food Loss and Waste Reduction”. Waste reduction is also highly relevant to “Responsible Consumption and Production”, no. 12 of the 17 Sustainable Development Goals (SDG) of the United Nations. Moreover, SDG no.2 is also addressed, namely “Zero Hunger” as the “Hidden Hunger” due to vitamin deficiency will be decreased.
Moreover, the project is part of the internationalization strategies of TUM as well as of USP. The project partner from USP has extensive experience in food microbiology, studying pathogenic and beneficial microbes in foods. Recent research of the group focused on the study and applications of lactic acid bacteria and probiotic strains capable of producing a variety of beneficial bioactive compounds, such as vitamins, proteolytic enzymes, bacteriocins and other antimicrobial peptides. The TUM group has long-standing experience in developing analytical methods for bioactive trace compounds, and in particular for folates. For folates, the TUM group developed a stable isotope dilution assay (SIDA), which includes all relevant vitamers and the polyglutamic forms. Besides analyzing the folate content in foods, the TUM group studied the folate bioavailability from foods in different human studies.
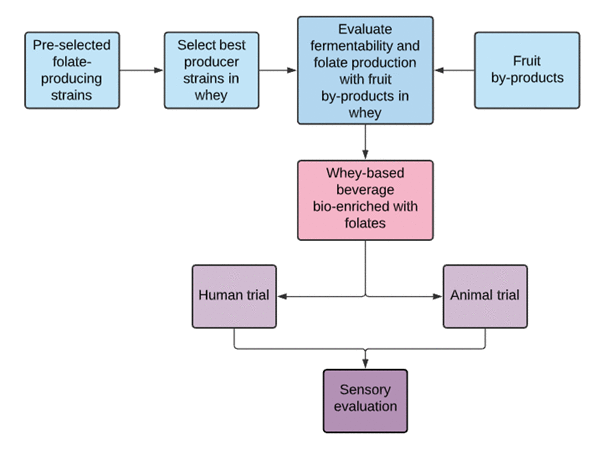
VITALAB consists of six different work packages (WP), of which the Chair of Analytical Food Chemistry will cover WP 2 (Accurate Quantitation of Total Folate and Folate Vitamer Distribution) and the human trial (WP 5) in cooperation with the ZIEL- Institute for Food & Health at TUM. Furthermore, the sensory evaluation (WP 6) will be conducted at the TUM. The group at the USP, by name Prof. Bernadette Dora Gombossy de Melo Franco, Prof. Susana Marta Isay Saad and M.Sc. Ana Clara Candelária Cucick will be responsible for WP 1 (Screening of lactic acid bacteria (LAB) for folate production) and the ooptimization of technological processes for maximum folate content (WP 3). Furthermore, the bioavailability of folates from the product in animal models (WP 4) will be determined under the supervision of the USP).
- WP 1: Screening of lactic acid bacteria (LAB) for folate production
Lactic acid bacteria will be tested for folate production in conventional microbiological culture media. Folate synthesis will then be tested on whey mixed with various industrial fruit by-products. Folate production is first determined by conventional microbiological analytical methods. The fruit by-products, selected according to the availability in the fruit industries in the State of Sao Paulo, arise from dragon fruit (pitahaya), passion fruit and grape processing.
- WP 2: Accurate quantitation of total folate and folate vitamer distribution
Stable isotope dilution assays (SIDA) using stable isotopologues of folate vitamers followed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis will be applied for the products with maximum folate content. Thus, accurate content determination of promising strain/substrate combinations will be achieved. This also allows determination of individual folate vitamers and prediction of folate bioavailability.
- WP 3: Optimization of technological processes for maximum folate content
The physical, chemical, and microbiological characteristics of whey and fruits by-products and the optimal time/temperature/pH conditions for the in situ folate production will be determined.
- WP 4: Assessing the bioavailability of folates from the product in animal models
Two protocols for the assessment of the bioavailability of folate in the products will be performed using mice. First, “Depletion-repletion” models, in which animals are initially submitted to a depletion of vitamins, simulating vitamin-deficient individuals, and second, “Prevention” models, in which animals are fed with the test-products along with a vitamin-deficient diet, simulating individuals consuming vitamin contents below the recommended daily dose.
- WP 5: Assessing the bioavailability of folates from the product in human studies
The bioavailability of folate in the products will be measured using human volunteers. In short-term studies the promising products high in folates will be tested by 6 volunteers. Blood plasma will be sampled, and plasma folate curves measured by SIDA.
- WP 6: Assessment of the sensorial properties of the generated products
The products presenting promising results in the animal and human models will be tested for sensorial acceptability.
Figure 1: Workflow of VITALAB.